The Pauli Exclusion Principle has its origin in the inability of two electrons in the same state of oscillation to interfere.
The Pauli Exclusion Principle makes the world “solid” in a sense that once atoms are there, they are restricted in occupying the same space. That introduces tangibility in the universe.
So far, there are only electromagnetic manifestations of the entities photon and neutrino, and these are not tangible. However, once the atom is build included the electron shells formed and filled, the phenomenon of tangibility comes into play when atoms collide. That is the basic phenomenon for the Pauli Exclusion Principle.
The definition of the Pauli Exclusion Principle is with quantum numbers.
Wikipedia:
The Pauli exclusion principle is the quantum mechanical principle which states that two or more identical fermions (particles with half-integer spin) cannot occupy the same quantum state within a quantum system simultaneously. In the case of electrons in atoms, it can be stated as follows: it is impossible for two electrons of a poly-electron atom to have the same values of the four quantum numbers: n, the principal quantum number, ℓ, the angular momentum quantum number, mℓ, the magnetic quantum number, and ms, the spin quantum number. For example, if two electrons reside in the same orbital, and if their n, ℓ, and mℓ values are the same, then their ms must be different, and thus the electrons must have opposite half-integer spin projections of 1/2 and −1/2. This principle was formulated by Austrian physicist Wolfgang Pauli in 1925 for electrons, and later extended to all fermions with his spin–statistics theorem of 1940.
In fact, most important is that two electrons in the same state of oscillation, cannot interfere.
Two electrons with opposite oscillation can as demonstrated by the so-called electron-positron annihilation.
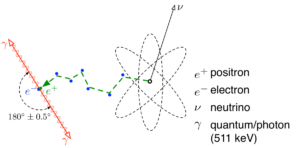
The Dutch Paradigm clarifies that a positron is not the anti-particle of the electron, but the same type of electron, but in opposite oscillation.
What in fact the Pauli Exclusion Principle implies, is that an electron-electron interference is not possible. From The postulates of The Dutch Paradigm, this makes sense. Such interference would breach the limit of the speed of light with the two neutrinos in the two electrons. It simply is not possible and therefore cannot happen.
The practical consequences require further study. There is no electron-positron annihilation when two atoms collide.
As explained in the chapter building the atom, the nucleus, and the electron shells, there is an intense tuning between the nucleus and the electrons in the shells. Therefore, prudence is required to avoid oversimplification.
