We now enter the realm of the macrocosmic world, by the forming of electron shells with electrons orbiting around the nucleus.
Much information is available regarding the electron shells. The Dutch Paradigm respects that information, but this new paradigm adds consequences on the phenomenon of electrons in orbits not yet recognized by regular science.
Electrons orbit around the nucleus at a speed of approximately 0.01 c. Each electron has a quant of free electric energy in its constituents that determine the frequency of the electromagnetic systems of the electron and ultimately the radius of its orbit.
Electrons in orbit interfere with the electric manifestations of a nucleus while orbiting through Coulomb’s force. The nucleus manifests a compounded asymmetric electrical manifestation. Such as described previously within The Dutch Paradigm, but there is a distinct difference with the prevailing paradigm.
The prevailing paradigm assumes that both the electron and the nucleus, have an isotropic manifestation of the “electric charge” of either + or – charge. The Dutch Paradigm indicates an asymmetric electrical manifestation that is anisotropic of nature and has equal character. These differences are consequential for the interference between the electron in orbit and the nucleus.
Let us consider the first element Hydrogen. It has 1 proton and ½ spin.
The electric vector of the electron in orbit points towards the electric vector of the proton bond. The electron and the proton are mutually attracting with the Coulomb force, while the electron in orbit is propagating at a high circular speed. Due to that circular movement, the electron induces a rotation of the nucleus around its axis perpendicular to the electric vector of the proton.
In regular science, this is not recognized, due to the of isotropy for the electric charge of the proton.
In as well the electron as the proton of the nucleus, energy was transferred in a sense that the electron forced the nucleus to start and maintain rotation, though with a small delay by creating a backlash that will induce the momentum to work and rotate against the inertia of the nucleus. An equilibrium in stable interference is established, under conservation of energy within the system electron in orbit, and the proton of the nucleus. The nucleus will follow a pattern that links into the inertia of the nucleus and the orbital speed of the electron.
The next nuclei under consideration are Deuterium and Tritium. These are isotopes of Hydrogen and have additional dodecahedrons. That modifies the interference variables as in the system just mentioned.
Helium has an electric charge with value 2. Its electric vectors in the nucleus point along two axes that are perpendicular. The additional electron will also orbit perpendicular to the first electron in the first electron shell. The nucleus now rotates around two axes and has higher levels of gyroscopic behavior.
The third electron is in the second shell. That electron triggers the third axis to rotate and from then onwards, we have a system that is gyroscopically working in the three Euclidean axes. It has a compounded complex of vibrations along three axes. That is a characteristic of the third element, being Lithium.
With more electrons and electron shells active, there is another effect. As from the third electron, a second shell houses an electron that will speed at approximately 0.01c. As a consequence, the nucleus will acquire higher levels of inertia and the electrons in the first shell will reduce in speed. Because the second shell is at a significantly larger diameter relative to the nucleus, the angular speed of rotation of the nucleus in its axes will react accordingly.
Due to the postulated anisotropic character of the electron, the nucleus reflects or mirror its composition towards the electrons in the subsequent shells. It has a specific vibrational response, magnetic behavior and so on. All in line with the specifics for the electrons that are the mirror image – though with a translation key – of its electric vector in the nucleus.
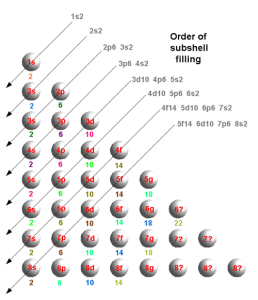
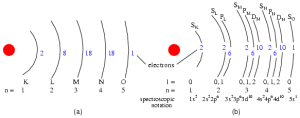
That leads to a set of shells as:
Alternatively, in a different format:
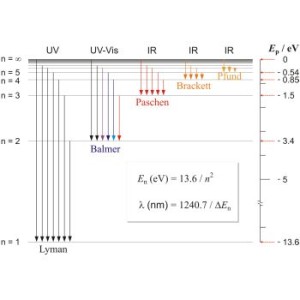
For these shells, there is much information in so-called Lyman , Balmer, Paschen, Brackett, and Pfund series.
The electrons in orbit oscillate with the frequency of approximately 10¹⁴ Hz and by doing so, rotate following the spinor functionality. This rotation maintains the position of attraction for the electron with Coulomb’s force with the nucleus.