We now enter the realm of the macrocosmic world by forming electron shells with electrons orbiting around the nucleus.
There is a wealth of information available about electron shells. The Dutch Paradigm acknowledges this information while recognizing that conventional science has assumed unsubstantiated properties of electrons in orbits.
Understanding the limitations of the assumed isotropic electric charge in both electrons and protons allows us to explore the implications of the new models presented in The Dutch Paradigm on our current concepts of electron shells.
The fundamental assumption for the model of the atom is based on the Bohr model. Although it is widely recognized that this model will eventually be replaced by a more advanced theory that addresses its inherent flaws, the Bohr model remains the most convenient foundation for understanding atomic behavior.
Electrons in orbit interact with the electric forces of a nucleus through Coulomb’s force. We can consider that a nucleus exhibits a complex, asymmetric electric manifestation in three dimensions. This perspective, as outlined in The Dutch Paradigm, represents a significant departure from the prevailing scientific understanding.
The Dutch Paradigm proposes an asymmetric electrical phenomenon that is anisotropic in nature while maintaining equal characteristics for both the electrons in orbit and the protons in the nucleus. These differences significantly affect the interaction between the orbiting electrons and the nucleus.
Let’s look at the first element, Hydrogen. It has 1 proton and a spin of 1/2.
The electric vector of the electron in orbit points towards the electric vector of the proton. The Coulomb force mutually attracts the electron and the proton while the electron in orbit moves at a high circular speed. Due to this circular movement, the electron causes the nucleus to rotate around its axis, perpendicular to the proton’s electric vector.
This interaction is not foreseen in standard physics.
Both the electron and the proton in the nucleus transfer energy in such a way that the electron prompts the nucleus to start and maintain its rotation. This process involves a slight delay due to a backlash effect, which generates momentum that counteracts the nucleus’s inertia, ultimately causing it to rotate. An equilibrium is established between the orbiting electron and the proton in the nucleus, which conserves energy within the system. The nucleus’s movement follows a pattern determined by its inertia and the orbital speed of the electron.
Helium has a electric ‘charge’ of +2. Its electric vectors can either align along the same axis or be positioned along two perpendicular axes. In the latter scenario, the additional electron orbits perpendicularly to the first electron within the first electron shell. Consequently, the nucleus rotates around two axes, demonstrating enhanced gyroscopic behavior.
The third electron resides in the second shell. This electron initiates the rotation of the third axis, resulting in a system that functions gyroscopically along the three Euclidean axes. This system exhibits a compounded complex of vibrations along these three axes, which is a characteristic of the third element, lithium.
As the number of electrons and electron shells increases, a new effect becomes apparent. When a third electron is added, it occupies a second shell, and this electron travels at approximately 0.01 times the speed of light. Consequently, the nucleus experiences higher levels of inertia, which causes the electrons in the first shell to slow down. Additionally, the second shell has a diameter relative to the nucleus, and the angular rotation speed of the nucleus will adjust accordingly on its axes.
In The Dutch Paradigm, the electron is thought to have an anisotropic nature, which means that the nucleus influences its composition in relation to the electrons in the subsequent shells. This interaction results in specific vibrational responses and magnetic behaviors that correspond to the unique characteristics of the electrons. These characteristics act as mirror images of the electric vector within the nucleus, though they are adjusted by a translation factor.
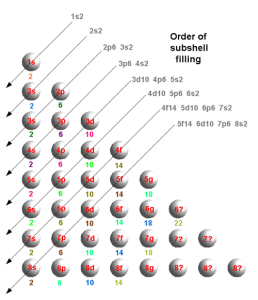
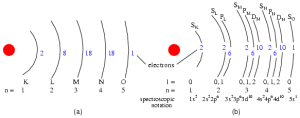
That leads to a set of shells as:
Alternatively, in a different format:
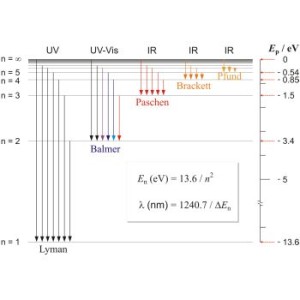
For these shells, there is much information available for the so-called Lyman , Balmer, Paschen, Brackett, and Pfund series.
In The Dutch Paradigm, electrons in orbit oscillate at a frequency of approximately 10¹⁴ Hz – the visible light spectrum, which falls within the visible light spectrum. They rotate according to spinor functionality while maintaining attraction to the nucleus through Coulomb’s force.
Accepting the postulate of anisotropy for electric manifestations, as seen in The Dutch Paradigm, opens up challenging areas for further exploration.